Modeling of jump of the inverse of the resistance of NaI-AgI solid electrolyte
Modelamiento del salto del inverso de la resistencia del electrolito sólido NaI-AgI

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
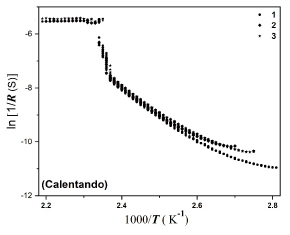
Electrical resistance measurements of NaI-AgI solid electrolyte showed that adding NaI to AgI stabilizes the transition from conducting ionic phase (β-AgI) to the superionic (α-AgI) at 420 K with increasing temperature. The ionic conductivity at the β → α transition of AgI increases by several orders of magnitude. Considering a phenomenological model based on a free energy density was fitted the abrupt jump of ionic conductivity. In this model, the equilibrium defect concentration (, R is the resistance) is the order parameter. Better results are achieved for the order parameter.
Article visits 308 | PDF visits 265
Downloads
- Agrawal, R. C. & Gupta, R. K., 1999. Review Superionic solids: composite electrolyte phase -an overview. J. Mat. Scie., Volume 34, p. 1131–1162. doi: 10.1023/A:1004598902146
- Burley, G., 1963. Polymorphism of silver iodide. American mineralogist. Volume 48, p. 1266–1276. https://pubs.geoscienceworld.org/ammin/article-pdf/48/11-12/1266/4254546/am-1963-1266.pdf
- Chandra, A., 2014. Ion conduction in crystalline superionic solids and its applications. Eur. Phys. J. Appl. Phys., 66 (30905 (pp 21)). doi: 10.1051/epjap/2014130569
- Chandra, S., 1981. Superionics Solids. Amsterdan: North-Holland.
- Huberman, A., 1974. Cooperative Phenomena in Solid Electrolytes. Phys. Rev. Lett., Volume 32, p. 1000–1002. doi 10.1103/PhysRevLett.32.1000. doi: 10.1103/PhysRevLett.32.1000
- Madden, P., O'Sullivan, K. F. & Chiarotti, G., 1992. Ordering of the silver ions in α-AgI: A mechanism for the α→β phase transition. Phy. Rev. B, 45(18), p. 10206–10212. doi: 10.1103/PhysRevB.45.10206
- Rice, M. J., Strässler, S. & Toombs, G. A., 1974. Superionic Conductors: Theory of the Phase Transition to the Cation Disordered State. Phys. Rev. Lett., Volume 32, p. 596. doi: 10.1103/PhysRevLett.32.596
- Rickert, H., 1978. Solid ionic conductors: principles and applications. Angew Chem Int Ed Engl, Volume 17, p. 37–46. doi: 10.1002/anie.197800371
- Siraj, K., 2012. Past, present and future of superionic conductors. Int. J. Nano Mater. Sci., Volume 1, pp. 1-20. https://www.researchgate.net/publication/235222104_Past_Present_and_Future_of_Superionic_Conductors
- Sunandana, C., 2016. Introduction to solid state ionics: phenomenology and applications. New York: CRC Press, Taylor & Francis.
- Welch, D. O. & Dienes, G. J., 1977. Phenomenological and microscopic models of sublattice disorder in ionic crystals -I Phenomenological models. J. Phys. Chem. Solids, Volume 38, p. 311–317.




