Modeling the dynamic viscosity of Newtonian Fluids using the Eyring’s Theory and the Residual Helmholtz Free Energy
Modelación de la viscosidad dinámica de fluidos newtonianos a partir de la teoría de Eyring y la energía libre de Helmholtz residual

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
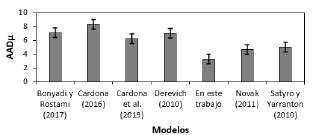
In this work, the dynamic viscosity of Newtonian fluids is modeled using Eyring's theory and the residual Helmholtz free energy. In order to estimate this energy, a modified Peng-Robinson cubic equation of state is applied. The adjustable parameters of the model have been determined from experimental data in the liquid and vapor coexistence for n-alkanes and n-alcohols. Later these parameters have been generalized using simple mathematical expressions that depend on the molecular weight of each substance. The predictive capabilities of the model are evaluated under single-phase conditions. The absolute deviations during correlation are below 3.27% while in prediction are below 5.60%. The generalized model is extended to mixtures using a simple mixture rule with one binary interaction parameter and the result without interaction is 8.19% and estimated is 3.45%. Finally, the model is compared with others in the literature and the statistical results show that the generalized model provides acceptable results.
Article visits 864 | PDF visits 447
Downloads
- Assael, M. J.; Charitidou, E.; Dymond, J. H.; Papadaki, M. (1992). Viscosity and thermal conductivity of binary n-heptane+ n-alkane mixtures. International journal of thermophysics, 13(2), 237-249. https://doi.org/10.1007/BF00504434
- Abdulagatov, I. M.; Azizov, N. D. (2006). (p, ρ, T, x) and viscosity measurements of {x1n-heptane+(1− x1) n-octane} mixtures at high temperatures and high pressures. The Journal of Chemical Thermodynamics, 38(11), 1402-1415. https://doi.org/10.1016/j.jct.2006.01.012
- Benabithe, Z. Z.; Vanegas, D.; Montoya, J. C. R.; Velásquez, J. A. (2020). Caso de estudio de la destilación etanol-agua en operación continua y discontinua y su simulación con ecuaciones cúbicas de estado y modelos de actividad. TecnoLógicas, 23(49), 223-249. https://doi.org/10.22430/22565337.1638
- Bonyadi, M.; Rostami, M. (2017). A new viscosity model based on Soave-Redlich-Kwong equation of state. Fluid Phase Equilibria, 451, 40-47. https://doi.org/10.1016/j.fluid.2017.07.009
- Bloxham, J. C.; Redd, M. E.; Giles, N. F.; Knotts IV, T. A.; Wilding, W. V. (2021). Proper Use of the DIPPR 801 Database for Creation of Models, Methods, and Processes. Journal of Chemical & Engineering Data, 66(1), 3-10. https://doi.org/10.1021/acs.jced.0c00641
- Cardona, L. F. (2016). Calculation of the viscosity of hydrocarbons based on the cubic equation of state of Jarrahian-Heidaryan. Revista Mexicana De Ingeniería Química, 15(3), 1019-1025.
- Cano-Gómez, J. J.; Iglesias-Silva, G. A.; Castrejón-González, E. O.; Ramos-Estrada, M.; Hall, K. R. (2015). Density and viscosity of binary liquid mixtures of ethanol+ 1-hexanol and ethanol+ 1-heptanol from (293.15 to 328.15) K at 0.1 MPa. Journal of
- Chemical & Engineering Data, 60(7), 1945-1955. https://doi.org/10.1021/je501133u
- Cardona, L. F.; Forero, L. A.; Velásquez, J. A. (2019). Modelamiento de la Viscosidad con Base en una Ecuación Cúbica μTP del Tipo Peng-Robinson. Información tecnológica, 30(4), 259-272. http://dx.doi.org/10.4067/S0718-07642019000400259
- Chevalier, J. L. E.; Petrino, P. J.; Gaston-Bonhomme, Y. H. (1990). Viscosity and density of some aliphatic, cyclic, and aromatic hydrocarbons binary liquid mixtures. Journal of Chemical and Engineering Data, 35(2), 206-212. https://doi.org/10.1021/je00060a034
- Daubert, T. E. (1998). Evaluated equation forms for correlating thermodynamic and transport properties with temperature. Industrial & Engineering Chemistry Research, 37(8), 3260-3267. https://doi.org/10.1021/ie9708687
- Derevich, I. V. (2010). Thermodynamic model of viscosity of hydrocarbons and their mixtures. International Journal of Heat and Mass Transfer, 53(19-20), 3823-3830. https://doi.org/10.1016/j.ijheatmasstransfer.2010.04.035
- Elliott, J. R.; Lira, C. T.; Lira, C. T. (2012). Introductory chemical engineering thermodynamics (2a ed.). Nueva York: Prentice Hall.
- Fan, T. B.; Wang, L. S. (2006). A viscosity model based on Peng-Robinson equation of state for light hydrocarbon liquids and gases. Fluid Phase Equilibria, 247(1-2), 59-69. https://doi.org/10.1016/j.fluid.2006.06.008
- Forero, L. A.; Velásquez, J. A. (2019). Representación Simultánea del Equilibrio Líquido-Vapor, el Volumen Molar y la Entalpía de Exceso de Mezclas Complejas mediante una Ecuación de Estado tipo Peng-Robinson. Información Tecnológica, 30(6), 21-34. http://dx.doi.org/10.4067/S0718-07642019000600021
- Hussein, N. M.; Asfour, A. F. A. (2009). Densities and kinematic viscosities of ten binary 1-alkanol liquid systems at temperatures of (293.15 and 298.15) K. Journal of Chemical & Engineering Data, 54(10), 2948-2952. https://doi.org/10.1021/je800497u
- Kontogeorgis, G. M.; Folas, G. K. (2010). Thermodynamic models for industrial applications: from classical and advanced mixing rules to association theories. Nueva York: John Wiley & Sons, Inc.
- Kumagai, A.; Yokoyama, C. (1998). Liquid viscosity of binary mixtures of methanol with ethanol and 1-propanol from 273.15 to 333.15 K. International Journal of Thermophysics, 19(1), 3-13. https://doi.org/10.1023/A:1021438800094
- Lasdon, L. S.; Waren, A. D.; Jain, A., Ratner, M. (1978). Design and testing of a generalized reduced gradient code for nonlinear programming. ACM Transactions on Mathematical Software, 4(1), 34-50. https://doi.org/10.1145/355769.355773
- Lemmon, E. W.; Huber, M. L.M.; Mclinden, M. O. (2007). NIST standard reference database 23: reference fluid thermodynamic and transport properties-REFPROP. (version 8.0) [software]. NIST-REFPROP 8. https://www.nist.gov/publications/nist-standard-reference-database-23-reference-fluid-thermodynamic-and-transport-0?pub_id=50520
- Liu, X.; Zhu, C.; He, M.; Zhang, Y. (2017). Correlation for viscosities of pure liquids at high pressures. Journal of Molecular Liquids, 231, 404-410. https://doi.org/10.1016/j.molliq.2017.02.026
- Martins, R. J.; Cardoso, M. J. D. M.; Barcia, O. E. (2003). A new model for calculating the viscosity of pure liquids at high pressures. Industrial & Engineering Chemistry Research, 42(16), 3824-3830. https://doi.org/10.1021/ie021017o
- Novak, L. T. (2011). Fluid viscosity-residual entropy correlation. International Journal of Chemical Reactor Engineering, 9(1), 1-27. https://doi.org/10.2202/1542-6580.2839
- Poulopoulos, S. G.; Philippopoulos, C. J. (2003). The effect of adding oxygenated compounds to gasoline on automotive exhaust emissions. J. Eng. Gas Turbines Power, 125(1), 344-350. https://doi.org/10.1115/1.1501076
- Poling, B. E.; Prausnitz, J. M.; O’connell, J. P. (2001). Properties of gases and liquids (5a ed.). Nueva York: McGraw-Hill Education.
- Sastry, N. V.; Raj, M. M. (1996). Densities, speeds of sound, viscosities, dielectric constants, and refractive indices for 1-heptanol+ hexane and+ heptane at 303.15 and 313.15 K. Journal of Chemical & Engineering Data, 41(3), 612-618. https://doi.org/10.1021/je950172p
- Sastry, N. V.; Valand, M. K. (1998). Densities, viscosities, and relative permittivities for pentane+ 1-alcohols (C1 to C12) at 298.15 K. Journal of Chemical & Engineering Data, 43(2), 152-157. https://doi.org/10.1021/je9701801
- Satyro, M. A.; Yarranton, H. W. (2010). Expanded fluid-based viscosity correlation for hydrocarbons using an equation of state. Fluid Phase Equilibria, 298(1), 1-11. https://doi.org/10.1016/j.fluid.2010.06.023
- Valderrama, J. O. (2003). The state of the cubic equations of state. Industrial & Engineering Chemistry Research, 42(8), 1603-1618. https://doi.org/10.1021/ie020447b
- Valderrama, J. O.; Muñoz, J. M.; Rojas, R. E. (2011). Viscosity of ionic liquids using the concept of mass connectivity and artificial neural networks. Korean Journal of Chemical Engineering, 28(6), 1451-1457. https://doi.org/10.1007/s11814-010-0512-0
- Varzandeh, F.; Stenby, E. H.; Yan, W. (2017). General approach to characterizing reservoir fluids for EoS models using a large PVT database. Fluid Phase Equilibria, 433, 97-111. https://doi.org/10.1016/j.fluid.2016.10.018
- Valderrama, J. O.; Cardona, L. F.; Rojas, R. E. (2019). Correlation and prediction of ionic liquid viscosity using Valderrama-Patel-Teja cubic equation of state and the geometric similitude concept. Part I: pure ionic liquids. Fluid Phase Equilibria, 497, 164-177. https://doi.org/10.1016/j.fluid.2019.04.034




