Study of the best conditions to determine pesticides in waters and sediments of the Cauca-Colombia River
Estudio de las mejores condiciones para determinar plaguicidas en aguas y sedimentos del Río Cauca-Colombia Conditions to determine pesticides in water and sediments

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
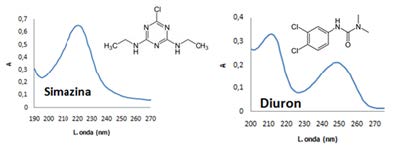
The different agricultural, industrial and economic activities generate pesticide residues that are reaching the different bodies of water, contaminating lakes, rivers, soils, biota, among others. In order to monitor these types of substances in the environment, it is necessary to adjust the analysis procedure in the best possible way. This work allowed to establish the best extraction and determination conditions of the pesticides simazina, atrazina, diuron, carbaril, propanil and metomil in river waters and sediments. Solid phase extraction (SPE) and ultrasound methods were used for the extraction of pesticides present in water and sediments respectively. The determination of the compounds was carried out by high performance liquid chromatography (HPLC-UV). The method was tested on water and sediment samples from the Cauca River as it passed through the Department of Valle del Cauca-Colombia-South America. Recovery percentages above 87% were obtained for five of the six pesticides extracted by SPE and above 80% for all pesticides extracted by ultrasound. The pesticides with the highest frequency found in the waters and sediments of the Cauca River were simazine, atrazine and diuron.
Article visits 460 | PDF visits 287
Downloads
- Amparán-Salido, R.T.; López Téllez, J.; Navarro Rodríguez, M. (2003). Metodologías para el estudio del impacto de contaminantes plaguicidas. RETEL revista de toxicológica en línea. pp. 15-18.
- Babic, S.; Petrovic, M.; Kastelan-Macan, M. (1998). Ultrasonic solvent extraction of pesticides from soil. Journal of Chromatography A, 823(1-2), pp. 3– 9. https://doi.org/10.1016/S0021-9673(98)00301-X
- Biswas, A.K.; Kondaiah, N.; Anjaneyulu, A.S.R.; Rao, G.S.; Singh, R.P. (2010). A simple assay for analyzing residues of carbaryl insecticide in buffalo meat by liquid chromatography–photodiode array detection. Analytical Methods, 2(4), pp. 393-396. https://doi.org/10.1039/B9AY00301K
- Carazo-Rojas, E.; Pérez-Rojas, G.; Pérez-Villanueva, M.; Chinchilla-Soto, C.; Chin-Pampillo, J.S.; Mora, P.; Alpízar-Marín, M.; Masís-Mora, M.; Rodríguez-Rodríguez, C.E.; Vryzas, Z. (2018). Pesticide monitoring and ecotoxicological risk assessment in surface water bodies and sediments of a tropical agro-ecosystem. Environmental Pollution, 241, pp. 800-809. https://doi.org/10.1016/j.envpol.2018.06.020
- Durak, B.Y.; Chormey, D.S.; Firat, M.; Bakirdere, S. (2020). Validation of ultrasonic-assisted switchable solvent liquid phase microextraction for trace determination of hormones and organochlorine pesticides by GC–MS and combination with QuEChERS. Food Chemistry, 305. https://doi.org/10.1016/j.foodchem.2019.125487.
- IUPAC. (1995). Nomenclature in Evaluation of Analytical Methods including Detection and Quantificaction Capabilities. Pure and Applied Chemistry, 67, 1699-1723.
- Cui, S.; Hough, R.; Yates, K.; Osprey, M.; Kerr, C.; Cooper, P.; Coull, M.; Zhang, Z. (2020). Effects of season and sediment-water exchange processes on the partitioning of pesticides in the catchment environment: Implications for pesticides monitoring. Science of The Total Environment, 698. https://doi.org/10.1016/j.scitotenv.2019.134228.
- Harshit, D.; Charmy, K.; Nrupesh, P. (2017). Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chemistry, 230, pp. 448-453. https://doi.org/10.1016/j.foodchem.2017.03.083
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. (2020). Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC Trends in Analytical Chemistry, 123. https://doi.org/10.1016/j.trac.2019.115772
- Masís, F.; Valdez, J.; Coto, T.; León, S. (2008). Residuos de agroquímicos en sedimentos de ríos, Poás, Costa Rica. Nota Técnica Agronomía Costarricense, 32(1), pp. 113-123.
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. (2018). Microbial Degradation of Organophosphate Pesticides: A Review. Pedosphere, 28(2), pp. 190-208. https://doi.org/10.1016/S1002-0160(18)60017-7.
- Nivia, E. (2000). Mujeres y plaguicidas, una mirada a la situación actual, tendencias y riesgos de los plaguicidas, estudio de caso en Palmira, Colombia, RAPALMIRA.
- Villamizar, M.L.; Brown, C.D. (2016). Modelling triazines in the valley of the River Cauca, Colombia, using the annualized agricultural non-point source pollution model. Agric. Water Manag. 177, pp. 24-36. https://doi.org/10.1016/j.agwat.2016.06.010.
- Pellicer-Castell, E.; Belenguer-Sapiña, C.; Amorós, P.; El Haskouri, J.; Herrero-Martínez, J.M.; Mauri-Aucejo, A.R. (2022). Mesoporous silica sorbent with gold nanoparticles for solid-phase extraction of organochlorine pesticides in water samples. Journal of Chromatography A. 1662. https://doi.org/10.1016/j.chroma.2021.462729.
- Petrie, B.; Camacho-Muñoz, D. (2021). Environmentally friendly analytical method to assess enantioselective behaviour of pharmaceuticals and pesticides in river waters. Sustainable Chemistry and Pharmacy, 24. https://doi.org/10.1016/j.scp.2021.100558.
- Pichon, V.; Coumes, C.C.D.; Chen, L.; Guenu, S.; Hennion, M.C. (1996). Simple removal of humic and fulvic acid interferences using polymeric sorbents for the simultaneous solid-phase extraction of polar acidic, neutral and basic pesticides. Chromatography A. 737, 25-33. https://doi.org/10.1016/0021-9673(95)01339-3
- RAS. (2000). Sistemas de potabilización. Reglamento Técnico del Sector de Agua Potable y Saneamiento Básico [Online]. Disponible en: https://procurement-notices.undp.org/view_file.cfm?doc_id=16483. Consultado: 22 febrero 2022.
- Rajput, S.; Kumari, A.; Arora, S.; Kaur, R. (2018). Multi-residue pesticides analysis in water samples using reverse phase high performance liquid chromatography (RP-HPLC). MethodsX. 5, pp. 744-751. https://doi.org/10.1016/j.mex.2018.07.005.
- Ruberu, S.R.; Draper, W.M.; Perera, S.K. (2000). Multiresidue HPLC methods for phenyl urea herbicides in water. J. Agric. Food Chem. 48. https://doi.org/10.1021/jf000266p
- Sarria-Villa, R.; Ocampo-Duque, W.; Páez, M.; Schuhmacher, M. (2016). Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Science of the Total Environment, 540, pp. 455 – 465. https://doi.org/10.1016/j.scitotenv.2015.07.020.
- Tao, S.; Guo, L.; Wang, X.; Liu, X.; Ju, T.; Dawson, R.; Cao, J.; Xu, F.; Li, B. (2004). Use of sequential ASE extraction to evaluate the bioavailability of DDT and its metabolites to wheat roots in soils with various organic carbon contents. Sci. Total Environ, 320, pp. 1 - 9. https://doi.org/10.1016/S0048-9697(03)00452-2.
- Tóth, E; Tölgyesi, A; Bálint, M; Ma, X; Sharma, V.K. (2022). Separation of fosetyl and phosphonic acid in food matrices with mixed-mode HPLC column coupled with tandem mass spectrometric detection and method application to other highly polar pesticides. Journal of Chromatography B, 1189. https://doi.org/10.1016/j.jchromb.2021.123083.
- USEPA. (2000). Enviromental Protection Agency.Method 3550 C. Ultrasonic Extraction [Online]. Disponible en: https://www.epa.gov/sites/default/files/2015-12/documents/3550c.pdf. Consultado: 10 enero 2022.
- Vagi, M.C.; Petsas, A.S.; Kostopoulou, M.N.; Karamanoli, M.K.; Lekkas T.D. (2007). Determination of organochlorine pesticides in marine sediments samples using ultrasonic solvent extraction followed by GC/ECD. Desalination, 210(1-3), pp. 146–156. https://doi.org/10.1016/j.desal.2006.06.020.




