Gold recovery in aqueous medium by cassava peels (Manihot esculenta) modified with citric acid
Recuperación de oro de soluciones acuosas por medio de cáscara de yuca (Manihot esculenta) modificada con ácido cítrico

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
Objective: to evaluate the performance of cassava peels modified with citric acid to recover the gold dissolved in synthetic solutions with concentrations similar to those found in the final processes of the gold production plants.
Methodology: Cassava peels were modified with citric acid by reacting the dried and milled peels with an aqueous solution of citric acid at 60 °C and sulfuric acid as catalysts, and subsequent heating at 150 °C for completion of the reaction. The synthetic solutions were preparing taking 0.5 g of metallic gold and dissolving it in a solution of 8 ml of HNO3 0.1 M; volume was completed until a solution of concentration in gold of 500 mg/L was reached. The influence of variables such as the initial gold concentration, temperature, and pH of the samples were analyzed in order to determine the optimum conditions in the recovery process. Experiments were carried out in Erlenmeyers in batch mode using an orbital shaker. The activation energy of the adsorption process was calculated as 157 + 14 kJ/mol. Gold removal values were found up to 99.58%, at a temperature of 45 ° C and pH of 10, these being optimal conditions.
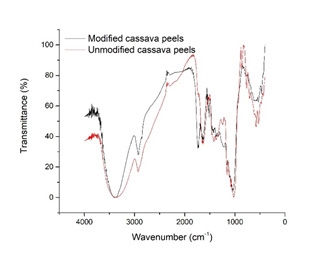
Results: FTIR and removal results revealed a successful modification of cassava peels. Kinetic and equilibrium data showed a good fitting to pseudo-second and Langmuir model, respectively. In general, adsorption capacity exhibited little dependency with temperature, but significant changes with pH. Gold removal values were found up to 99.58%, at a temperature of 45 ° C and pH of 10, these being optimal conditions.
Conclusions: Cassava peel modified with citric acid showed and excellent performance as adsorbent of gold from aqueous acid and basic solutions with removal percentages higher than other biomasses and the unmodified cassava peels.
Article visits 489 | PDF visits 190
Downloads
- Acosta Arguello, H. A.; Barraza Yance, C. A.; Albis Arrieta, A. R. (2017). Adsorption of chromium (VI) using cassava peel (Manihot esculenta) as biosorbent: A kinetic study. Ingeniería y Desarrollo, 35(1), pp. 58–76. https://doi.org/10.14482/inde.35.1.8943
- Albis, A.; Martínez, J.; Severiche, M.; Garcia, J. (2016). Remoción de plomo de soluciones acuosas usando cáscara de yuca modificada con ácido cítrico. Avances Investigación En Ingeniería, 13(2). https://doi.org/10.18041/1794-4953/avances.2.254
- Albis, A. R.; Cajar R, L.; Domínguez, M. I. (2015). Análisis cinético de la adsorción de Cr (VI) en soluciones acuosas a concentraciones de 10-20 mg/L con el uso de cáscara de yuca amarga (Manihot esculenta). Prospectiva, 13(2), pp. 64. https://doi.org/10.15665/rp.v13i2.488
- Albis Arrieta, A. R.; Arzuza Orellano, S. A.; Mosquera PAlacio, A. M. (2019). Remoción de Mercurio (II) en solución acuosa usando residuo industrial de yuca (Manihot esculenta). Prospectiva, 17(2). https://doi.org/10.15665/rp.v17i2.1951
- Albis Arrieta, A. R.; Martínez, J.; Santiago, P. (2017). Remoción de Zinc (II) de soluciones acuosas usando cáscara de yuca (Manihot esculenta): Experimentos en columna/Removal of zinc (II) from aqueous solutions using cassava peel (Manihot esculenta): column experiments. Prospectiva, 15(1), pp.16–28. https://doi.org/10.15665/rp.v15i1.773
- Albis Arrieta, A. R.; Ortiz Toro, J. D.; Martínez De la Rosa, J. E. (2017). Remoción de cromo hexavalente de soluciones acuosas usando cáscara de yuca (Manihot esculenta): Experimentos en columna. INGE CUC, 13(1), pp. 42–52. https://doi.org/10.17981/ingecuc.13.1.2017.04
- Araújo, C. S. T.; Almeida, I. L. S.; Rezende, H. C.; Marcionilio, S.; Léon, J. J. L.; de Matos, T. N. (2018). Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchemical Journal, 137, pp. 348–354. https://doi.org/10.1016/j.microc.2017.11.009
- Bustos, M.; Ibarra, H.; Dussán, J. (2018). The Golden Activity of Lysinibacillus sphaericus: New Insights on Gold Accumulation and Possible Nanoparticles Biosynthesis. Materials, 11(9), pp. 1587. https://doi.org/10.3390/ma11091587
- Choudhary, B. C.; Paul, D.; Borse, A. U.; Garole, D. J. (2018). Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Separation and Purification Technology, 195, pp. 260–270. https://doi.org/10.1016/j.seppur.2017.12.024
- Cristiano, E.; Hu, Y.-J.; Siegfried, M.; Kaplan, D.; Nitsche, H. (2011). A Comparison of Point of Zero Charge Measurement Methodology. Clays and Clay Minerals, 59(2), pp. 107–115. https://doi.org/10.1346/CCMN.2011.0590201
- Das, N. (2010). Recovery of precious metals through biosorption — A review. Hydrometallurgy, 103(1–4), 180–189. https://doi.org/10.1016/j.hydromet.2010.03.016
- do Nascimento, J. M.; Cruz, N. D.; de Oliveira, G. R.; Sá, W. S.; de Oliveira, J. D.; Ribeiro, P. R. S.; Leite, S. G. F. (2021). Evaluation of the kinetics of gold biosorption processes and consequent biogenic synthesis of AuNPs mediated by the fungus Trichoderma harzianum. Environmental Technology & Innovation, 21, pp. 101238. https://doi.org/10.1016/j.eti.2020.101238
- Fonseca, J.; Albis, A.; Montenegro, A. R. (2018). Evaluation of zinc adsorption using cassava peels (Manihot esculenta) modified with citric acid. Contemporary Engineering Sciences, 11(72), pp. 3575–3585. https://doi.org/10.12988/ces.2018.87364
- Garside, M. (2021). Leading gold exporting countries worldwide in 2020 [Online]. Available on: https://www.statista.com/statistics/298446/gold-exports-of-major-countries/#statisticcontainer.
- Girado, L.; Moreno, J. C. (2006). Relación entre la entalpía de inmersión de un carbón activado en soluciones acuosas de Pb2+ y los parámetros de adsorción. Revista Colombiana de Química, 35(1), pp. 41–49.
- Jorgetto, A. O.; Silva, R. I. V.; Saeki, M. J.; Barbosa, R. C.; Martines, M. A. U.; Jorge, S. M. A.; Silva, A. C. P.; Schneider, J. F.; Castro, G. R. (2014). Cassava root husks powder as green adsorbent for the removal of Cu(II) from natural river water. Applied Surface Science, 288, pp. 356–362. https://doi.org/10.1016/j.apsusc.2013.10.032
- Leal Esper, Y. E. (2019). Minería ilegal, conflicto armado y vulneración al medio ambiente. Revista Infometric@ -Serie Ciencias Sociales y Humana, 2(1), pp. 21–43.
- Lodeiro, P.; Sillanpää, M. (2013). Gold reduction in batch and column experiments using silica gel derivates and seaweed biomass. Chemical Engineering Journal, 230, pp. 372–379. https://doi.org/10.1016/j.cej.2013.06.105
- Martínez Bautista, I. (2008). Adsorción y formación de nanoparticulas de oro y plata en microorganismos aislados de residuos mineros, tesis (maestría en ciencias en desarrollo sostenible), México, Tecnológico de Monterrey. http://hdl.handle.net/11285/628534
- Ortiz-Riomalo, J. F.; Rettberg, A. (2018). Minería de oro, conflicto y criminalidad en los albores del siglo en Colombia: Perspectivas para el posconflicto colombiano. Colombia Internacional, 93, 17–63. https://doi.org/10.7440/colombiaint93.2018.02
- Petrov, N.; Budinova, T.; Razvigorova, M.; Ekinci, E.; Yardim, F.; Minkova, V. (2000). Preparation and characterization of carbon adsorbents from furfural. Carbon, 38(15), pp. 2069–2075. https://doi.org/10.1016/S0008-6223(00)00063-4
- Salminen, J.; Blomberg, P.; Mäkinen, J.; Räsänen, L. (2015). Environmental aspects of metals removal from waters and gold recovery. AIChE Journal, 61(9), pp. 2739–2748. https://doi.org/10.1002/aic.14917
- Simate, G. S.; Ndlovu, S.; Seepe, L. (2015). Removal of heavy metals using cassava peel waste biomass in a multi-stage countercurrent batch operation. Journal of the Southern African Institute of Mining and Metallurgy, 115(12), pp. 1137–1141. https://doi.org/10.17159/2411-9717/2015/v115n12a1
- Syed, S. (2012). Recovery of gold from secondary sources—A review. Hydrometallurgy, 115–116, pp. 30–51. https://doi.org/10.1016/j.hydromet.2011.12.012
- Tien, C.; Ramarao, B. (2017). On the significance and utility of the Lagergren model and the pseudo second-order model of batch adsorption. Separation Science and Technology, 52(6), pp. 975–986. https://doi.org/10.1080/01496395.2016.1274327
- Tran, H. N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. (2017). Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Research, 120, pp. 88–116. https://doi.org/10.1016/j.watres.2017.04.014
- Vargas, C.; Navarro, P.; Araya, E.; Pavez, F.; Alguacil, F. J. (2006). Recuperación de oro a partir de disoluciones de amoniaco y tiosulfato utilizando carbón activado. Revista de Metalurgia, 42(3), pp. 222–233. http://revistademetalurgia.revistas.csic.es/index.php/revistademetalurgia/article/view/22
- Wu, F.-C.; Tseng, R.-L.; Huang, S.-C.; Juang, R.-S. (2009). Characteristics of pseudo-second-order kinetic model for liquid-phase adsorption: A mini-review. Chemical Engineering Journal, 151(1–3), pp. 1–9. https://doi.org/10.1016/j.cej.2009.02.024
- Zhou, Y.; Zhu, N.; Kang, N.; Cao, Y.; Shi, C.; Wu, P.; Dang, Z.; Zhang, X.; Qin, B. (2017). Layer-by-layer assembly surface modified microbial biomass for enhancing biorecovery of secondary gold. Waste Management, 60, pp. 552–560. https://doi.org/10.1016/j.wasman.2016.12.015




