Silver nanoparticles from Borojoa patinoi and their in vitro effect on Candida albicans biofilms
Nanopartículas de plata de Borojoa patinoi y su efecto in vitro sobre biopelículas de Candida albicans Effect of nanoparticles on biofilms

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
Introduction
Candida albicans is capable of generating infections related to medical devices by colonizing them and forming biofilms. Conventional treatments fail because they cannot efficiently enter the biofilm.
Methods
Silver nanoparticles (NPsAg) from Borojoa patinoi were prepared by the green synthesis method and their effect on pre-formed and post-formed Candida albicans biofilm was evaluated by performing MTT, and microscopy visualization of Gram and White stains. Calcofluor and Scanning Electron Microscopy (SEM).
Results
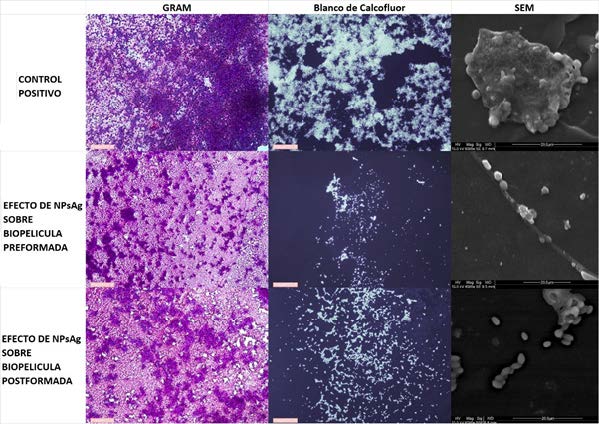
Green NPsAg with a spherical shape and a size of 20-60nm were generated by synthesis. The sessile minimum inhibitory concentration 50 and 80 of NPsAg was >2 µg/mL on pre-formed and established biofilms of Candida albicans. Microscopically under all staining and Scanning Electron Microscopy we detect the reduction of pre-formed biofilms and inhibition in the formation of new biofilms when treated with NPsAg. By SEM, changes in the yeast wall were observed.
Conclusions
Borojoa patinoi nanoparticles were found to inhibit biofilm formation and destroy already established biofilms. We observed the deformation in the wall of C. albicans yeasts by SEM when subjected to NPsAg.
Article visits 273 | PDF visits 166
Downloads
- Busi, S. and Rajkumari, J. (2019) ‘Microbially synthesized nanoparticles as next generation antimicrobials: scope and applications’, Nanoparticles in Pharmacotherapy. 2019/04/12, pp. 485–524. doi:10.1016/b978-0-12-816504-1.00008-9.
- Candida albicans, inside the oral cavity’, Artif Cells Nanomed Biotechnol. 2015/05/15, 44(6), pp. 1429–1433. doi:10.3109/21691401.2015.1031907.
- Chaves-López, C. et al. (2018) ‘Potential of Borojoa patinoi Cuatrecasas water extract to inhibit nosocomial antibiotic resistant bacteria and cancer cell proliferation in vitro’, Food Funct. 2018/04/17, 9(5), pp. 2725–2734. doi:10.1039/c7fo01542a.
- Chung, I.M. et al. (2016) ‘Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications’, Nanoscale Res Lett. 2016/01/29, 11(1), p. 40. doi:10.1186/s11671-016-1257-4.
- Dakal, T.C. et al. (2016) ‘Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles’, Frontiers in microbiology, 7, p. 1831. doi:10.3389/fmicb.2016.01831.
- Estevez, M.B. et al. (2020) ‘Biofilm Eradication Using Biogenic Silver Nanoparticles’, Molecules (Basel, Switzerland), 25(9), p. 2023. doi:10.3390/molecules25092023.
- Gómez, M. (2018) ‘Nanomateriales, Nanopartículas y Síntesis verde’, Repertorio de Medicina y Cirugía, 27(2), pp. 75–80.
- Gómez-Garzón, M. et al. (2021) ‘Inhibition of the filamentation of Candida albicans by Borojoa patinoi silver nanoparticles’, SN Applied Sciences, 3(2), p. 195. doi:10.1007/s42452-020-04103-0.
- Huang, Y.W., Cambre, M. and Lee, H.J. (2017) ‘The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms’, Int J Mol Sci. 2017/12/14, 18(12). doi:10.3390/ijms18122702.
- Kim, K.J. et al. (2009) ‘Antifungal activity and mode of action of silver nano-particles on Candida albicans’, Biometals. 2008/09/05, 22(2), pp. 235–242. doi:10.1007/s10534-008-9159-2.
- Kumari, P., Luqman, S. and Meena, A. (2019) ‘Application of the combinatorial approaches of medicinal and aromatic plants with nanotechnology and its impacts on healthcare’, Daru. 2019/05/28, 27(1), pp. 475–489. doi:10.1007/s40199-019-00271-6.
- Lara, H.H. et al. (2015) ‘Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study’, J Nanobiotechnology, 13, p. 91.
- Le Mauff, F. (2020) ‘Exopolysaccharides and Biofilms’, Curr Top Microbiol Immunol. 2020/02/20, 425, pp. 225–254. doi:10.1007/82_2020_199.
- Lizcano, L.J. et al. (2010) ‘Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use’, Food Chemistry, 119(4), pp. 1566–1570. doi:https://doi.org/10.1016/j.foodchem.2009.09.043.
- Lukaszuk, C., Krajewska-Kulak, E. and Kulak, W. (2017) ‘Retrospective observation of drug susceptibility of Candida strains in the years 1999, 2004, and 2015’, PeerJ, 5, p. e3038.
- Magana, M. et al. (2018) ‘Options and limitations in clinical investigation of bacterial biofilms’, Clinical Microbiology Reviews, 31(3), pp. 1–49. doi:10.1128/CMR.00084-16.
- Monteiro, D.R. et al. (2011) ‘Silver colloidal nanoparticles: antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata’, Biofouling. 2011/07/16, 27(7), pp. 711–719. doi:10.1080/08927014.2011.599101.
- Mousavi, S.M. et al. (2018) ‘Green synthesis of silver nanoparticles toward bio and medical applications: review study’, Artif Cells Nanomed Biotechnol. 2018/10/18, 46(sup3), pp. S855–S872. doi:10.1080/21691401.2018.1517769.
- Pathog. 2010/04/03, 6(3), p. e1000828. doi:10.1371/journal.ppat.1000828.
- Patra, J.K., Shin, H.-S. and Das, G. (2021) ‘Characterization and Evaluation of Multiple Biological Activities of Silver
- Nanoparticles Fabricated from Dragon Tongue Bean Outer Peel Extract’, International journal of nanomedicine, 16, pp. 977–987. doi:10.2147/ijn.s290037.
- Pierce, C.G. et al. (2008) ‘A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing’, Nat Protoc. 2008/09/06, 3(9), pp. 1494–1500. doi:10.1038/nport.2008.141.
- Rajendran, R. et al. (2016) ‘Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012-2013’, Clin Microbiol Infect. 2015/10/04, 22(1), pp. 87–93. doi:10.1016/j.cmi.2015.09.018.
- Römling, U. and Balsalobre, C. (2012) ‘Biofilm infections, their resilience to therapy and innovative treatment strategies’, J Intern Med. 2012/10/03, 272(6), pp. 541–561. doi:10.1111/joim.12004.
- Selvaraj, M. et al. (2014) ‘Highly potential antifungal activity of quantum-sized silver nanoparticles against Candida albicans’, Appl Biochem Biotechnol. 2014/03/22, 173(1), pp. 55–66. doi:10.1007/s12010-014-0782-9 [doi].
- Seneviratne, C.J., Jin, L. and Samaranayake, L.P. (2008) ‘Biofilm lifestyle of Candida: a mini review’, Oral Diseases, 14(7), pp. 582–590. doi:https://doi.org/10.1111/j.1601-0825.2007.01424.x.
- Silva, S. et al. (2017) ‘Candida Species Biofilms’ Antifungal Resistance’, J Fungi (Basel), 3(1).
- Sotelo D, I., Casas F, N. and Camelo M, G. (2010) ‘BOROJÓ (Borojoa patinoi): FUENTE DE POLIFENOLES CON ACTIVIDAD ANTIMICROBIANA’, Vitae, 17, pp. 329–336. Available at: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121-40042010000300011&nrm=iso.
- Uppuluri, P. et al. (2010) ‘Dispersion as an important step in the Candida albicans biofilm developmental cycle’, PLoS
- Vallabhaneni, S. et al. (2016) ‘The Global Burden of Fungal Diseases’, Infectious Disease Clinics of North America, 30(1), pp. 1–11. doi:https://doi.org/10.1016/j.idc.2015.10.004.
- Wang, H. and Xie, B. (2016) ‘Study on how nanosilver-based inorganic antibacterial agent functions on biofilm formation of




