Modeling and parametrization of an adsorption column for nickel removal using computer-aided process engineering
Modelado y parametrización de una columna de adsorción para la remoción de níquel utilizando ingeniería de procesos asistida por computador

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
Heavy metals are pollutants that are generated by different activities, one of which is the
dumping of wastewater by industries into bodies of water, which represents a great threat
to aquatic and terrestrial biota, as well as health. These contaminants are persistent,
bioaccumulative and non-biodegradable, generating a negative effect on the food chain in the
area of influence. Nickel is a heavy metal that is used in different types of industries such
as battery production. This generates different harmful effects on the human body, such as
the cardiovascular or digestive system when exposed in large quantities. The objective of the
present study is to use Computer Aided Process Engineering (CAPE) to model an operational
column on an industrial scale aimed at the adsorption of Nickel (II) in aqueous solution taking
advantage of the biomass of Theobroma cacao L. Consequently, Aspen Adsorption software
was used to carry out multiple simulations of an adsorption column using various industrial
configurations, with the aim of performing a parametric sensitivity analysis. In the results
obtained, it is evident that the Langmuir model - global linear resistance kinetic model (LDF)
used to simulate the adsorption column in the elimination of Nickel (II) achieves efficiencies
of up to 95.8%. The best conditions for the simulation in the adsorption column were an
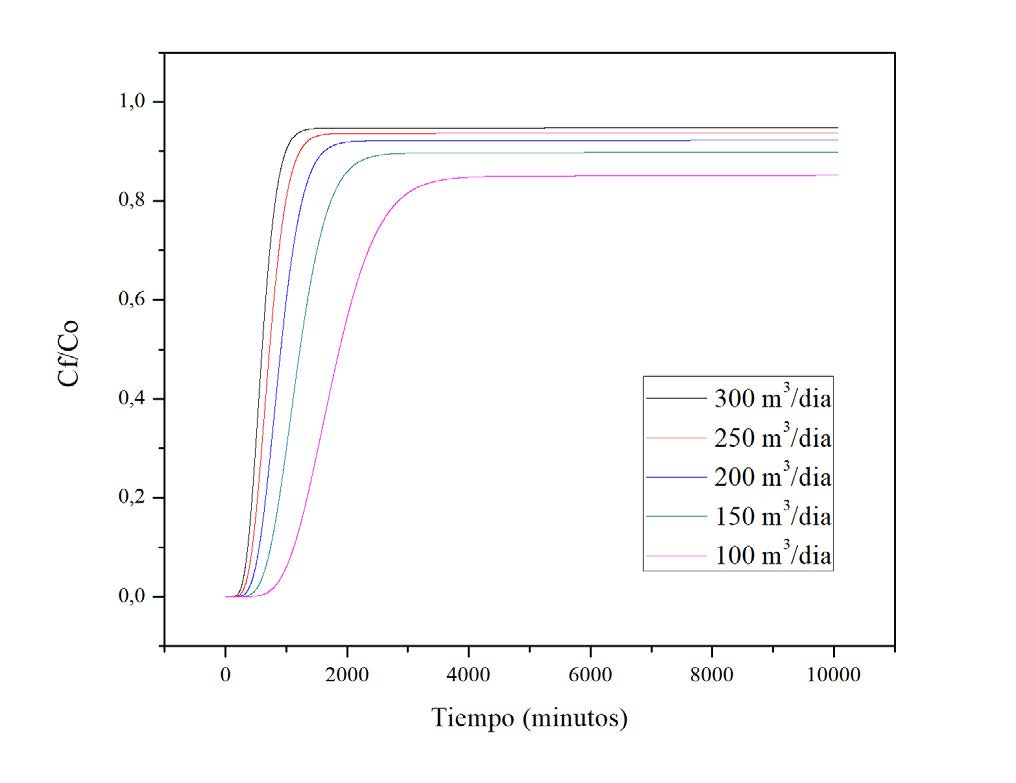
inlet flow rate of 300 m3/day, a bed height of 5 m and an initial concentration of 2000 mg/L.
Furthermore, it was observed that increasing the inlet flow led to a decrease in the rupture
and saturation time of the process, while increasing the bed height presented an increase in
the rupture and saturation time. On the other hand, concentration did not significantly affect
the efficiency of the process.
Article visits 186 | PDF visits 96
Downloads
- Agarwal, A., Upadhyay, U., Sreedhar, I. & Anitha, K. L. (2022) 'Simulation studies of Cu(II) removal from aqueous solution using olive stone', Cleaner Materials, 5, p. 100128. doi: 10.1016/j.clema.2022.100128.
- Ahmed, S., Unar, I. N., Khan, H. A., Maitlo, G., Mahar, R. B., Jatoi, A. S., Memon, A. Q. & Shah, A. K. (2020) 'Experimental study and dynamic simulation of melanoidin adsorption from distillery effluent', Environmental Science and Pollution Research, 27(9), pp. 9619–9636. doi: 10.1007/s11356-019-07441-8.
- Ali Abd, A. & Roslee Othman, M. (2022) 'Biogas upgrading to fuel grade methane using pressure swing adsorption: Parametric sensitivity analysis on an industrial scale', Fuel, 308, p. 121986. doi: 10.1016/j.fuel.2021.121986.
- Almazán-Ruiz, F. J., Caballero, F., Cruz-Díaz, M. R., Rivero, E. P., Vazquez-Arenas, J. & González, I. (2015) 'Nickel recovery from an electroplating rinsing effluent using RCE bench scale and RCE pilot plant reactors: The influence of pH control', Chemical Engineering Research and Design, 97, pp. 18–27. doi: 10.1016/j.cherd.2015.02.022.
- Benyahia, F. & O’Neill, K. E. (2005) 'Enhanced voidage correlations for packed beds of various particle shapes and sizes', Particulate Science and Technology, 23(2), pp. 169–177. doi: 10.1080/02726350590922242.
- Biswal, B. K. & Balasubramanian, R. (2023) 'Use of biochar as a low-cost adsorbent for removal of heavy metals from water and wastewater: A review', Journal of Environmental Chemical Engineering, 11(5), p. 110986. doi: 10.1016/j.jece.2023.110986.
- Chen, X., Hossain, M. F., Duan, C., Lu, J., Tsang, Y. F., Islam, M. S. & Zhou, Y. (2022) 'Isotherm models for adsorption of heavy metals from water - A review', Chemosphere, 307, p. 135545. doi: 10.1016/J.CHEMOSPHERE.2022.135545.
- Dixon, A. G. (1988) 'Correlations for wall and particle shape effects on fixed bed bulk voidage', The Canadian Journal of Chemical Engineering, 66(5), pp. 705–708. doi: 10.1002/cjce.5450660501.
- Fang, Y., Liu, L., Xiang, H., Wang, Y. & Sun, X. (2022) 'Biomass-based carbon microspheres for removing heavy metals from the environment: a review', Materials Today Sustainability, 18, p. 100136. doi: 10.1016/J.MTSUST.2022.100136.
- González-Delgado, Á. D., Moreno-Sader, K. A. & Martínez-Consuegra, J. D. (2022) Biorrefinación sostenible del camarón: desarrollos desde la ingeniería de procesos asistida por computador. UNIMINUTO. doi: 10.26620/uniminuto/978-958-763-558-4.
- González-Delgado, Á. D., Tejada-Tovar, C. & Villabona-Ortíz, A. (2022) 'Parametric Sensitivity Analysis of Chromium (Vi) Adsorption using Theobroma Cacao L Biomass via Process Simulation', Chemical Engineering Transactions, 92, pp. 535–540. doi: 10.3303/CET2292090.
- Guan, W., Tian, S., Cao, D., Chen, Y. & Zhao, X. (2017) 'Electrooxidation of nickel-ammonia complexes and simultaneous electrodeposition recovery of nickel from practical nickel-electroplating rinse wastewater', Electrochimica Acta, 246, pp. 1230–1236. doi: 10.1016/j.electacta.2017.06.121.
- Guan, Z., Guo, Y., Huang, Z., Liao, X., Chen, S., Ou, X., Sun, S., Liang, J., Cai, Y., Xie, W. & Xian, J. (2022) 'Simultaneous and efficient removal of organic Ni and Cu complexes from electroless plating effluent using integrated catalytic ozonation and chelating precipitation process in a continuous pilot-scale system', Chemical Engineering Journal, 428, p. 131250. doi: 10.1016/J.CEJ.2021.131250.
- Hardyianto Vai Bahrun, M., Kamin, Z., Anisuzzaman, S. M., Bono, A. & Bahrun, M. H. V. (2021) 'Assessment of adsorbent for removing lead (pb) ion in an industrial-scaled packed bed column', Journal of Engineering Science and Technology, 16(2), pp. 1213–1231.
- Jmiai, A., El Hayaoui, W., El-Asri, A., Skotta, A., Elhayaoui, W., Tamimi, M., Assabbane, A. & El Issami, S. (2023) 'Suspended matter and heavy metals (Cu and Zn) removal from water by coagulation/flocculation process using a new Bio-flocculant: Lepidium sativum', Journal of the Taiwan Institute of Chemical Engineers, 145, pp. 1876–1070. doi: 10.21203/rs.3.rs-1792666/v1.
- Juela, D., Vera, M., Cruzat, C., Astudillo, A. & Vanegas, E. (2022) 'A new approach for scaling up fixed-bed adsorption columns for aqueous systems: A case of antibiotic removal on natural adsorbent', Process Safety and Environmental Protection, 159, pp. 953–963. doi: 10.1016/j.psep.2022.01.046.
- Júnior, C. J. C. & Pessoa Filho, P. de A. (2023) 'Modeling and simulation of an industrial adsorption process of dehydration of natural gas in 4A molecular sieves: Effect of adsorbent aging', Results in Engineering, 18, p. 101144. doi: 10.1016/j.rineng.2023.101144.
- Koua, B. K., Koffi, P. M. E. & Gbaha, P. (2019) 'Evolution of shrinkage, real density, porosity, heat and mass transfer coefficients during indirect solar drying of cocoa beans', Journal of the Saudi Society of Agricultural Sciences, 18(1), pp. 72–82. doi: 10.1016/j.jssas.2017.01.002.
- Kumar, A., Kumar, V., Thakur, M., Bakshi, P., Koul, A., Javaid, A., Radziemska, M. & Pandey, V. C. (2023) 'Comprehensive review of nickel biogeochemistry, bioavailability, and health risks in the environment', Land Degradation and Development, 34(14), pp. 4141–4156. doi: 10.1002/ldr.4775.
- Kumar, V. & Kumar, M. (2022) Integrated Environmental Technologies for Wastewater Treatment and Sustainable Development. ELSEVIER. doi: 10.1016/C2020-0-04469-5.
- Lara, J., Tejada, C., Villabona, Á., Arrieta, A. & Granados Conde, C. (2016) 'Adsorción de plomo y cadmio en sistema continuo de lecho fijo sobre residuos de cacao', Revista ION, 29(2), pp. 113–124. doi: 10.18273/REVION.V29N2-2016009.
- Marcantonio, V., Bocci, E., Ouweltjes, J. P., Del Zotto, L. & Monarca, D. (2020) 'Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus', International Journal of Hydrogen Energy, 45(11), pp. 6651–6662. doi: 10.1016/j.ijhydene.2019.12.142.
- Musah, M., Azeh, Y., Mathew, J., Umar, M., Abdulhamid, Z. & Muhammad, A. (2022) 'Adsorption Kinetics and Isotherm Models: A Review', Caliphate Journal of Science and Technology, 4(2), pp. 230–243.
- Naiya, T. K., Bhattacharya, A. K. & Das, S. K. (2009) 'Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina', Journal of Colloid and Interface Science, 333(1), pp. 14–26. doi: 10.1016/j.jcis.2009.01.011.
- Qin, L., Xia, T., Li, X., Wu, X., Xie, W., Li, L., Zou, B., Guo, S. & He, T. (2023) 'Ni2+ adsorption on a new biochar from rice husk hydrochar and its excellent reuse in different simulated nickel plating wastewater', Journal of Environmental Management, 325, p. 116493. doi: 10.1016/j.jenvman.2022.116493.
- Rehman, A., Ihsanullah, I. & Atieh, M. A. (2021) 'A novel magnetic carbon composite derived from lignocellulosic biomass as a potential adsorbent for the removal of ciprofloxacin', Environmental Science and Pollution Research, 28(30), pp. 40206–40218. doi: 10.1007/s11356-021-13373-6.
- Saif, M. S., Abdulkareem, M. S., Jawad, A. H., Ajeel, M. A., Nomanbhay, S. & Ghaffar, S. H. A. (2022) 'Development of biomass-based activated carbon as an adsorbent from sago palm bark waste for Ni(II) adsorption', International Journal of Environmental Science and Technology, 19(10), pp. 10043–10058. doi: 10.1007/s13762-021-03738-w.
- Tejada-Tovar, C., González-Delgado, Á. D., Olmos-Roldán, G. & Sarmiento-Suárez, J. A. (2021) 'Comparison of adsorption kinetic models for heavy metals (Cr(VI), Hg(II) and Pb(II)) on agro-industrial biomass from Theobroma cacao and Bactris guineensis', Contemporary Engineering Sciences, 14(1), pp. 19–29. doi: 10.12988/ces.2021.91405.
- Tejada-Tovar, C., Olmos-Roldán, G., Villabona-Ortíz, Á. & González-Delgado, Á. D. (2019) 'Optimization of continuous adsorption of lead and chromium on biomass from avocado shell and seed', Ingeniería y Competitividad, 21(1), pp. 121–130. doi: 10.25100/iyc.v21i1.6852.
- Tejada-Tovar, C., Rojas, J. L., Villabona-Ortíz, Á. & González-Delgado, Á. D. (2020) 'Experimental and theoretical investigation on kinetic adsorption models for lead and cadmium removal onto rice husk biomass', Iranian Journal of Chemistry and Chemical Engineering (IJCCE), 39(4), pp. 135–148. doi: 10.30492/ijcce.2020.117062.3295.
- Tejada-Tovar, C., Villabona-Ortíz, Á., Jaimes, J. A. & González-Delgado, Á. D. (2022) 'Cu(II) adsorption via agroindustrial bioadsorbents: Isotherm, thermodynamic and dynamic modeling', Contemporary Engineering Sciences, 15(1), pp. 19–34. doi: 10.12988/ces.2022.91902.
- Tejada-Tovar, C., Villabona-Ortíz, Á., Rojas, J. L. & González-Delgado, Á. D. (2022) 'Adsorption isotherm and dynamic behavior of lead(II) onto avocado peel and seed', International Journal of Environmental Science and Technology, 19(6), pp. 5101–5116. doi: 10.1007/s13762-021-03558-y.
- Tong, X., Liu, W., Jin, X., Li, C., Yang, Q., Zeng, G., Hu, J., Chen, J., Hu, J., Chen, J., Deng, C. & Li, X. (2019) 'Activated biochar derived from iron-impregnated distillers grain for methylene blue removal: Adsorption performance and mechanism studies', Bioresource Technology, 274, pp. 1–9. doi: 10.1016/j.biortech.2018.11.091.
- Tran, T. K. N., Bui, T. T. & Vu, M. T. (2018) 'Studies on the adsorption of Pb2+ by an industrial by-product using response surface methodology', Journal of Environmental Chemical Engineering, 6(4), pp. 5089–5097. doi: 10.1016/j.jece.2018.07.013.
- Treviño-Cordero, H., Juárez-Aguilar, L. G., Mendoza-Castillo, D. I., Hernández-Montoya, V., Montes-Morán, M. A. & Bonilla-Petriciolet, A. (2013) 'Synthesis and characterization of activated carbons from coconut shell impregnated with FeCl3', Comptes Rendus Chimie, 16(2), pp. 173–182. doi: 10.1016/j.crci.2012.09.010.
- Zhu, X., Wang, Y., Zhu, D., Wei, Q., Wang, Y. & Yang, J. (2022) 'Enhancing nickel adsorption capacity of biochar derived from herbal medicine residue by magnetic modification', Journal of Environmental Management, 324, p. 116365. doi: 10.1016/j.jenvman.2022.116365.
- Zulfikar, M. A. & Setiyanto, H. (2013) 'Comparison of adsorption equilibrium, kinetics and thermodynamics of Pb(II) and Cr(III) on chitosan and modified chitosan', Carbohydrate Polymers, 97(2), pp. 425–432. doi: 10.1016/j.carbpol.2013.05.015.




