Calcium precursor effect on structural and microstructural properties of hydroxyapatite
Efecto del precursor de calcio en las propiedades estructurales y microestructurales de la hidroxiapatita

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright statement
The authors exclusively assign to the Universidad EIA, with the power to assign to third parties, all the exploitation rights that derive from the works that are accepted for publication in the Revista EIA, as well as in any product derived from it and, in in particular, those of reproduction, distribution, public communication (including interactive making available) and transformation (including adaptation, modification and, where appropriate, translation), for all types of exploitation (by way of example and not limitation : in paper, electronic, online, computer or audiovisual format, as well as in any other format, even for promotional or advertising purposes and / or for the production of derivative products), for a worldwide territorial scope and for the entire duration of the rights provided for in the current published text of the Intellectual Property Law. This assignment will be made by the authors without the right to any type of remuneration or compensation.
Consequently, the author may not publish or disseminate the works that are selected for publication in the Revista EIA, neither totally nor partially, nor authorize their publication to third parties, without the prior express authorization, requested and granted in writing, from the Univeridad EIA.
Show authors biography
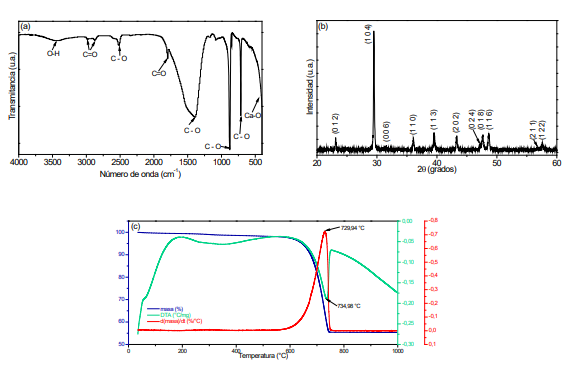
Nowadays the bone conditions have been a meaning clinical challenge and solutions are limited, sometimes ineffective. Hydroxyapatite investigation (main bone component) has gained significant importance. In this research, was analyzed the calcium precursor effect on structural and microstructural properties of hydroxyapatite comparing results of hydroxyapatite obtained from a natural source. Through the solution combustion synthesis were synthesized hydroxyapatite powders using calcium carbonate extracted from eggshell and commercial calcium carbonate and calcium nitrate. As well the natural source of hydroxyapatite was bovine bone which was washed, fractured and heat treatment. The functional groups were obtained by infrared spectroscopy and the crystalline phases by X-ray diffraction. Transmission electron microscopy allowed to determine the particle spherical morphology produced from calcium carbonate (eggshell) with the smallest size (∼20-50 nm) while those obtained by commercial precursors presented nonhomogeneous morphology. The results showed that the respective process followed was efficient to get hydroxyapatite nanoparticles obtained from calcium carbonate at temperature of 1100ºC. Calcium carbonate from eggshells allowed getting HAp whose morphology was homogeneous with nanometric size.
Article visits 386 | PDF visits 303
Downloads
- Abere, D.V.; Ojo, S.A.; Oyatogun, G.M.; Paredes-Epinosa, M.B.; Dharsika Niluxsshun, M.C.; Hakami, A. (2022). Mechanical and morphological characterization of nano-hydroxyapatite (nHA) for bone regeneration: A mini review. Biomedical Engineering Advances, 4, 100056. https://doi.org/10.1016/j.bea.2022.100056
- Bahloul, L.; Azzi, A.; Maradi, H. (2020): Study of The Porosity and Density of Synthetically Produced Hydroxyapatite. SAJ Biotechnology, 7, 1, pp. 1-5.
- Bantikatla, H.; N.S.M.P., Latha Devi; Bhogoju, R.K. (2021). Microstructural parameters from X-ray peak profile analysis by Williamson-Hall models; A review. Materials Today: Proceedings, 47(14), pp. 4891-4896. https://doi.org/10.1016/j.matpr.2021.06.256
- Barrett, E.P.; Brown, J.M.; Oleck, S.M. (1951). Some granular carbonaceous adsorbents for sugar refining. Industrial & Engineering Chemistry Research., 43(3), pp. 639-654. https://doi.org/10.1021/ie50495a026
- Chen, L.J.; Chen, T.; Cao, J.; Liu B.L.; Shao, C.S; Zhou, K.C.; Zhang, D. (2018). Effect of Tb/Mg doping on composition and physical properties of hydroxyapatite nanoparticles for gene vector application. Transactions of Nonferrous Metals Society of China, 28(1), pp. 125-136. https://doi.org/10.1016/S1003-6326(18)64645-X
- De Carvalho, B.; Rompen, E.; Lecloux, G.; Schupbach, P.; Dory, E.; Art, J. F.; Lambert, F. (2019). Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite. Materials (Basel), 12(23), 3946. https://doi.org/10.3390/ma12233946
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. (2018). Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regenerative Biomaterials, 5(4), pp. 197–211. https://doi.org/10.1093/rb/rby013
- Desai, K.R.; Alone, S.T.; Wadgane, S.R.; Shirsath, S.E.; Batoo, K.M.; Imran, A.; Raslan, E.H.; Hadi, M.; Ijaz, M.F.; Kadam, R.H. (2021). X-ray diffraction-based Williamson–Hall analysis and rietveld refinement for strain mechanism in Mg–Mn co-substituted CdFe2O4 nanoparticles. Physica B: Condensed Matter, 614, 413054. https://doi.org/10.1016/j.physb.2021.413054
- Diningsih, C.; Rohmawati, L. (2022). Synthesis of Calcium Carbonate (CaCO3) from Eggshell by Calcination Method. Indonesian Physical Review, 5(3), pp. 208-215.
- Dorozhkin, S.V. (2013). A detailed history of calcium orthophosphates from 1770s till 1950. Materials Science and Engineering: C, 33(6), pp. 3085-3110. https://doi.org/10.1016/j.msec.2013.04.002
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. (2022). Analysis of combustion synthesis method for Cu/CeO2 synthesis by integrating thermodynamics and design of experiments approach. Results in Engineering, 15, 100574. https://doi.org/10.1016/j.rineng.2022.100574
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. (2021). Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics, 4, pp. 542-563. https://doi.org/10.3390/ceramics4040039
- Frikha, K.; Limousy, L.; Bouaziz, J.; Bennici, S.; Chaari, K.; Jeguirim, M. (2019). Elaboration of alumina-based materials by solution combustion synthesis: A review. Comptes Rendus Chimie, 22(2-3), pp. 206-219. https://doi.org/10.1016/j.crci.2018.10.004
- Gandou, Z.; Nounah, A.; Belhorma, B.; Yahyaoui, A. (2015). Nanosized Calcium-Deficient Carbonated Hydroxyapatite synthesized by microwave activation. Journal of Materials and Environmental Science, 6(4), pp. 983-988
- Hu, C.; Ashok, D.; Nisbet, D. R.; Gautam, V. (2019). Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials, 219, 119366. https://doi.org/10.1016/j.biomaterials.2019.119366
- Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Wahap, M.A. (2022). Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon, 8(8), e10356. https://doi.org/10.1016/j.heliyon.2022.e10356
- Irfan, H.; Racik, K; Anand, S. (2018). Microstructural evaluation of CoAl2O4 nanoparticles by Williamson–Hall and size–strain plot methods. Journal of Asian Ceramic Societies, 6(1), pp. 54-62. https://doi.org/10.1080/21870764.2018.1439606
- Jain, A.; Somvanshi, A.; Prashant; Ahmad, N. (2023).X-ray diffraction analysis of SrTiO3 nanoparticles by Williamson-Hall, size-strain plot and FullProf method. Materials Today: Proceedings, in press. https://doi.org/10.1016/j.matpr.2023.03.166
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. (2019). Bioactive calcium phosphate materials and applications in bone regeneration. Biomaterials research, 23, 4. https://doi.org/10.1186/s40824-018-0149-3
- Kalita, S.J.; Bhatt, H.A. (2007). Nanocrystalline hydroxyapatite doped with magnesium and zinc, Synthesis and characterization. Materials Science and Engineering, 27(4), pp. 837-848. https://doi.org/10.1016/j.msec.2006.09.036
- Kalpana, M.; Nagalakshmi, R. (2023). Effect of reaction temperature and pH on structural and morphological properties of hydroxyapatite from precipitation method, Journal of the Indian Chemical Society, 100, 100947. https://doi.org/10.1016/j.jics.2023.100947
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. (2017). New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Annals of Anatomy - Anatomischer Anzeiger, 213, pp. 83-90. https://doi.org/10.1016/j.aanat.2017.05.010
- Le, B.Q.; Nurcombe, V.; Cool, S.M.; van Blitterswijk, C.A.; de Boer, J.; LaPointe, V.L.S. (2017). The Components of Bone and What They Can Teach Us about Regeneration. Materials (Basel), 11(1), 14. https://doi.org/10.3390/ma11010014
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. (2006). Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochimica Acta 447(1), pp. 115-120. https://doi.org/10.1016/j.tca.2006.04.013
- Mohd Pu'ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. (2019). Syntheses of hydroxyapatite from natural sources. Heliyon, 5(5), e01588. https://doi.org/10.1016/j.heliyon.2019.e01588
- Mohd Pu'ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z., Idris, M.I.; Lee, T.C. (2020) Synthesis method of hydroxyapatite: A review. Materials Today: Proceedings, 29(1), pp. 233-239. https://doi.org/10.1016/j.matpr.2020.05.536
- Nunes, J.P.; Neme, N.P.; de Souza Matos, M.J.; Junio, R.; Batista, C.; de Almeida Macedo, W.A.; Gastelois, P.L.; Gomes, D.A.; Rodrigues, M.A.; Cipreste, M.F.; Barros Sousa, E.M. (2023). Nanostructured system based on hydroxyapatite and curcumin: a promising candidate for osteosarcoma therapy. Ceramics International, In Press, Journal Pre-proof, https://doi.org/10.1016/j.ceramint.2023.03.115
- Omori, Y.; Okada, M.; Takeda, S.; Matsumoto, N. (2014). Fabrication of dispersible calcium phosphate nanocrystals via a modified Pechini method under non-stoichiometric conditions. Materials Science and Engineering, 42, pp. 562-568. https://doi.org/10.1016/j.msec.2014.05.071
- Peña, J. (2003). Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. Journal of the European Ceramic Society, 23(10), pp. 1687-1696. https://doi.org/10.1016/S0955-2219(02)00369-2
- Puspitasari, P.; Utomo, D.M.; Zhorifah, H.N.; Permanasari, A.A.; Gaya, R.W. (2020). Physicochemical Determination of Calcium Carbonate (CaCO3) from Chicken Eggshell. Key Engineering Materials, 840, pp. 478-483. https://doi.org/10.4028/www.scientific.net/KEM.840.478
- Qiao, D.; Cheng, S.; Xing, Z.; Zhang, Q.; Song, S.; Yan, F.; Zhang, Y. (2023). Bio-inspired glycosylated nano-hydroxyapatites enhance endogenous bone regeneration by modulating macrophage M2 polarization. Acta Biomaterialia, 162, pp. 135-148. https://doi.org/10.1016/j.actbio.2023.03.027
- Rh Owen, G.; Dard, M.; Larjava, H. (2018). Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. Journal of biomedical materials research. Part B, Applied biomaterials, 106(6), pp. 2493–2512. https://doi.org/10.1002/jbm.b.34049
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. (2013). Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta biomaterialia, 9(8), pp. 7591–7621. https://doi.org/10.1016/j.actbio.2013.04.012
- Saxena, V.; Pandey, L.M. (2022). Synthesis and Sintering of Calcium Hydroxyapatite for Biomedical Applications, Editor(s): M.S.J. Hashmi, Encyclopedia of Materials: Plastics and Polymers, Elsevier, 859-870. https://doi.org/10.1016/B978-0-12-820352-1.00136-X
- Venkateswarlu, K.; Sandhyarani, M.; Nellaippan, T.A.; Rameshbabu, N. (2014). Estimation of Crystallite Size, Lattice Strain and Dislocation Density of Nanocrystalline Carbonate Substituted Hydroxyapatite by X-ray Peak Variance Analysis. Procedia Materials Science, 5, pp. 212-221. https://doi.org/10.1016/j.mspro.2014.07.260
- Zhan, J.; Tseng, Y.H.; Chan, J.C.C.; Mou, C.Y. (2005). Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Advanced Functional Materials, 15, pp. 2005–2010. https://doi.org/10.1002/adfm.200500274




